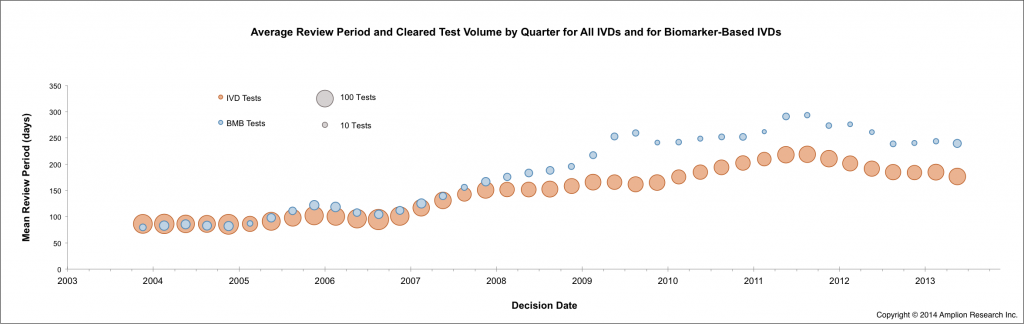
As previously reported in this blog, review periods for biomarker-based IVD tests were shorter in 2013 for the second year in a row. This continued the reversal of a decade-long trend of increasing review periods.
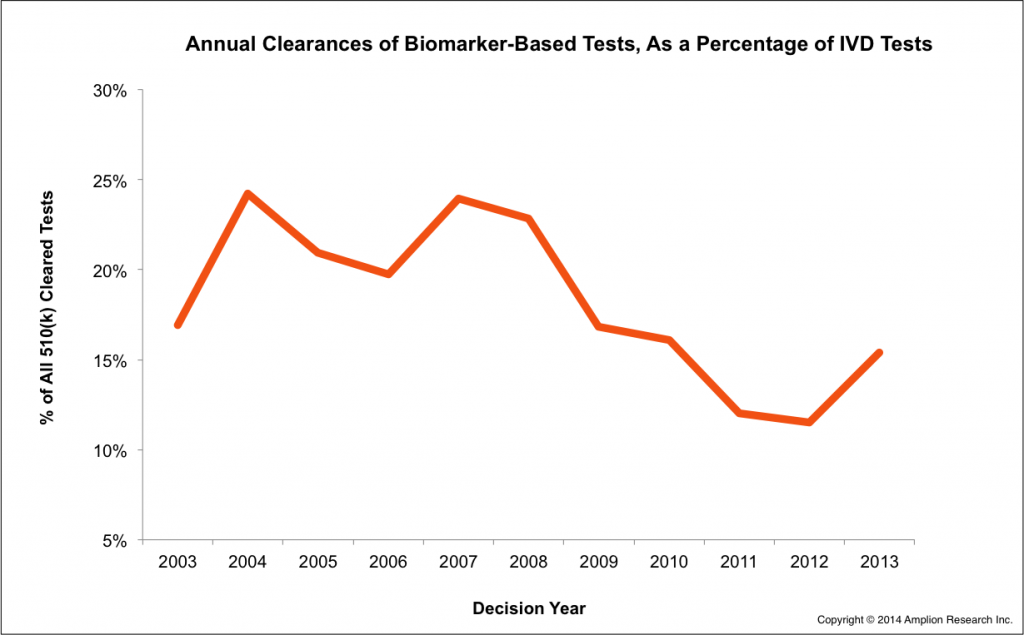
We can also now report that more biomarker-based tests were cleared by 510(k) in 2013 than in previous years, and in fact biomarker-based tests represented over 15% of all IVD tests cleared in 2013.
The average review period for biomarker-based tests is still longer than for IVD tests overall (as shown in the figure below), but the difference is shrinking compared to 2011 and 2012.
While lab-developed tests (LDTs) are likely to remain the dominant test type in common medical use, at least for now, FDA-cleared tests seem to be holding their own. In future posts we will refresh our analyses of the targets and indications being measured with tests approved in 2013, to see if anything can help to explain any changing roles for FDA-cleared tests relative to LDTs.
If you are interested in a full analysis of biomarker-based test trends, please download our free report, Commercial Trends for Biomarker-Based IVD Tests (2003-2012). The 2013 data will be included in a forthcoming reissue of the report, but the current issue contains much useful information.